DOS/PDOS of Au2Te12(-4) Anion
Oxidation state of Au in (Et4N)4Au2Te12
from the viewpoint of the interaction between the Au3+ and [Te12]10- ions, each "Au3+" ion of the [Au2Te12]4- anion receives about 2.8 electrons from the Te atoms directly coordinated with it. Therefore, the most reasonable oxidation state of Au in the [Au2Te12]4- anion is Au+ (d10), i.e., all the d-block levels of Au are completely filled in this anion. To explore the above finding further, it is necessary to examine where the d-block levels of Au lie in the energy spectrum of the [Au2Te12]4- anion. For this purpose, it is convenient to examine the TDOS plot for the [Au2Te12]4- anion and the PDOS plot for the d-orbitals of the Au atoms. The following figure presents such a plot calculated for the [Au2Te12]4- anion, where the TDOS is represented by a solid line, and the PDOS of the Au d-orbitals by a dashed line. It is clear that the d-block levels of the Au atoms lie well below the Fermi level, i.e., the highest occupied level of the [Au2Te12]4- anion.
Obviously, the observation reflects the fact that the 5d-level of Au lies considerably lower than the 5p-level of Te. (Namely, -17.6 vs. -9.73 eV in the CRMM Basis set, -14.27 vs. -9.79 eV in the ADF Basis set, and -15.08 vs. -13.2 eV in the Collected Basis set.) The present calculation is based on the Collected Basis set, which provides the smallest energy difference between the Au 5d and the Te 5p levels. The user may repeat calculations after decreasing this energy difference further (by either raising the Te 5p level or lowering the Au 5d level). For any reasonable variation of the VSIP values of the Au 5d and the Te 5p levels, the user will find that the above observation remains unchanged. Thus, with some confidence, we may conclude that not only in the [Au2Te12]4- anion but also in other binary compounds of Au and Te, the Au atoms should be monovalent.
from the viewpoint of the interaction between the Au3+ and [Te12]10- ions, each "Au3+" ion of the [Au2Te12]4- anion receives about 2.8 electrons from the Te atoms directly coordinated with it. Therefore, the most reasonable oxidation state of Au in the [Au2Te12]4- anion is Au+ (d10), i.e., all the d-block levels of Au are completely filled in this anion. To explore the above finding further, it is necessary to examine where the d-block levels of Au lie in the energy spectrum of the [Au2Te12]4- anion. For this purpose, it is convenient to examine the TDOS plot for the [Au2Te12]4- anion and the PDOS plot for the d-orbitals of the Au atoms. The following figure presents such a plot calculated for the [Au2Te12]4- anion, where the TDOS is represented by a solid line, and the PDOS of the Au d-orbitals by a dashed line. It is clear that the d-block levels of the Au atoms lie well below the Fermi level, i.e., the highest occupied level of the [Au2Te12]4- anion.
Obviously, the observation reflects the fact that the 5d-level of Au lies considerably lower than the 5p-level of Te. (Namely, -17.6 vs. -9.73 eV in the CRMM Basis set, -14.27 vs. -9.79 eV in the ADF Basis set, and -15.08 vs. -13.2 eV in the Collected Basis set.) The present calculation is based on the Collected Basis set, which provides the smallest energy difference between the Au 5d and the Te 5p levels. The user may repeat calculations after decreasing this energy difference further (by either raising the Te 5p level or lowering the Au 5d level). For any reasonable variation of the VSIP values of the Au 5d and the Te 5p levels, the user will find that the above observation remains unchanged. Thus, with some confidence, we may conclude that not only in the [Au2Te12]4- anion but also in other binary compounds of Au and Te, the Au atoms should be monovalent.
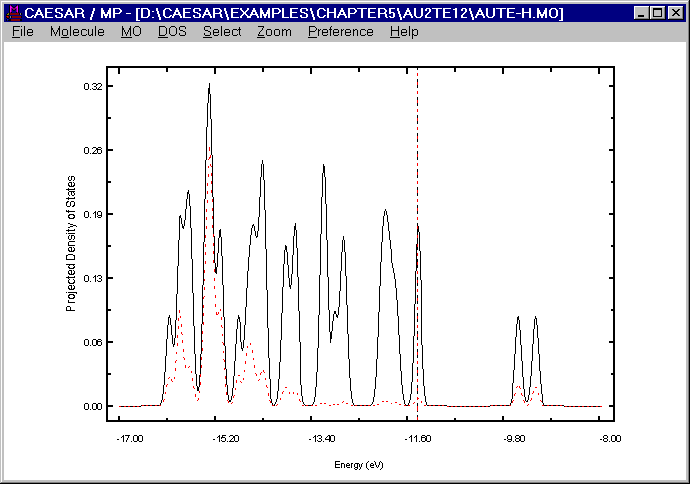
Figure. DOS and PDOS plots for [Au2Te12]4- anion.
Go back to The Gallery